Mitochondrial dysfunction and protective cellular responses
Cellular responses to mitochondrial defects
Mitochondrial proteostasis is critical for cellular health. During ageing, mitochondrial activity declines and dysfunction of has been linked to a variety of human diseases. Proteostasis is mainly maintained by a network of mitochondrial chaperones and proteases, that assist in protein folding and degrade non-functional or superfluous proteins. When mitochondrial quality control systems fail, the resulting proteomic imbalances are sensed by the cell and trigger protective cellular responses to restore and maintain organellar integrity. The best characterized are nuclear transcriptional changes that result in increased expression of chaperones and proteases to restore mitochondrial proteostasis. However, several aspects of the protective mitochondrial responses are incompletely understood: Which stress models reflect physiologically relevant mitochondrial defects? What is the signal that indicates mitochondrial dysfunction to the rest of the cell? Which transcription factors mediate the nuclear transcriptional response and how are newly synthesized chaperones and proteases imported into functionally compromised mitochondria?
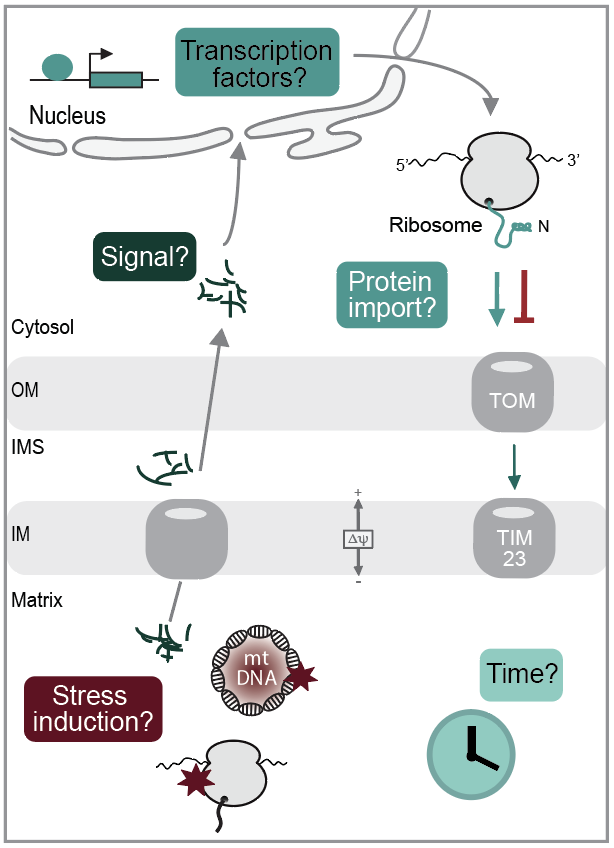
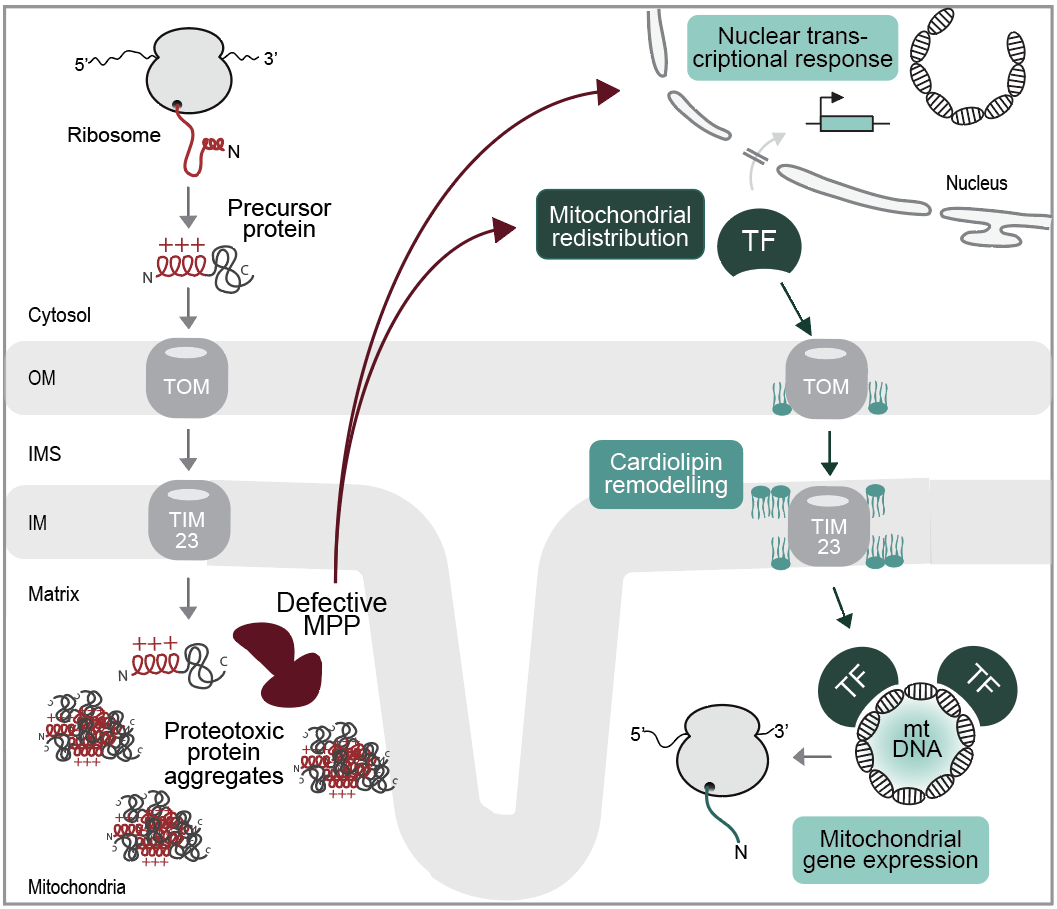
We are using stress models in yeast and human cell lines derived from patient mutations in the mitochondrial presequence proteases. Insertion of the point mutations into the respective proteases results in the accumulation of unprocessed precursors within the mitochondrial matrix, forming proteotoxic protein aggregates. Transcriptomic profiling revealed a rapid transcriptional response to the mitochondrial aggregates. Using this patient-based stress model, we found that mitochondrial integrity is strictly depending on the translocation of the nuclear transcription factor Rox1 into mitochondria, where it binds to mitochondrial DNA and maintains mitochondrial gene expression. Rox1 import is supported by lipidomic changes: The mitochondrial signature lipid Cardiolipin increases upon mitochondrial stress and binds tightly to the membrane translocases required for mitochondrial protein import. Current projects focus on the regulation of mitochondrial stress responses by cytosolic signalling networks and aim to dissect the different levels of protective responses in human cell lines.