Mitochondrial biogenesis and protein quality control
The initial cytosolic steps in mitochondrial protein biogenesis
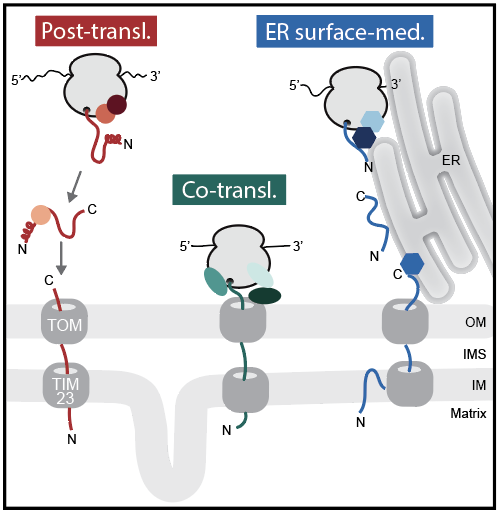
The vast majority of the mitochondrial proteome is synthesized in the cytosol and imported into mitochondria by translocases. While protein import into mitochondria is thought to be predominantly post-translational, ribosomes detected at the mitochondrial surface suggest that co-translational mitochondrial protein import also occurs. The recent identification of an ER surface-mediated protein import pathway adds another layer of complexity. We aim to analyze the initial cytosolic events in mitochondrial protein biogenesis using global in vivo profiling techniques to monitor the cytosolic import pathways and identify their substrate spectrum. Furthermore, we want to investigate the modulation of these cytosolic targeting processes upon mitochondrial stress by identifying the targeting factors and chaperones that perform cytosolic triage of mitochondrial precursors to prevent their toxic accumulation and overloading of the cytosolic proteostasis network.
Protein maturation upon import
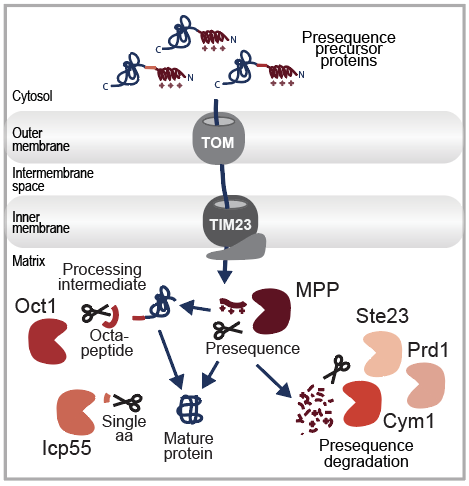
After crossing the mitochondrial membranes, the majority of mitochondrial precursors require proteolytic processing to become functional. The maturation step removes the mitochondrial targeting signal, the N-terminal presequence, which appr. 70% of all mitochondrial precursor proteins use to direct their import into the organelle. Using global N-proteomic approaches, we identified the substrate spectrum of the main Mitochondrial Processing Protease (MPP) and identified a secondary cleavage event required to stabilize the newly imported proteins. For this quality control, the Intermediate Cleaving Peptidase 55 (Icp55) or the OCTapeptidylaminopeptidase 1 (Oct1) removes a single amino acid or an additional octapeptide. The cleaved presequences and octapeptides are then degraded by the peptidases Cym1, Ste23 and Prd1. Together, these proteases form the presequence processing machinery and ensure proper protein maturation and hence proteome stability.
Dysfunction of presequence proteases causes severe human diseases such as neurodegeneration and cardiomyopathies. While we have a good understanding of the physiological relevance of presequence processing, we now want to understand the consequences of its dysfunction. To this end, we are introducing patient mutations into homologous yeast proteins and human cell lines to analyze the pathophysiological effects of patient mutations in molecular detail.
Dysfunction of presequence proteases causes severe human diseases such as neurodegeneration and cardiomyopathies. While we have a good understanding of the physiological relevance of presequence processing, we now want to understand the consequences of its dysfunction. To this end, we are introducing patient mutations into homologous yeast proteins and human cell lines to analyze the pathophysiological effects of patient mutations in molecular detail.
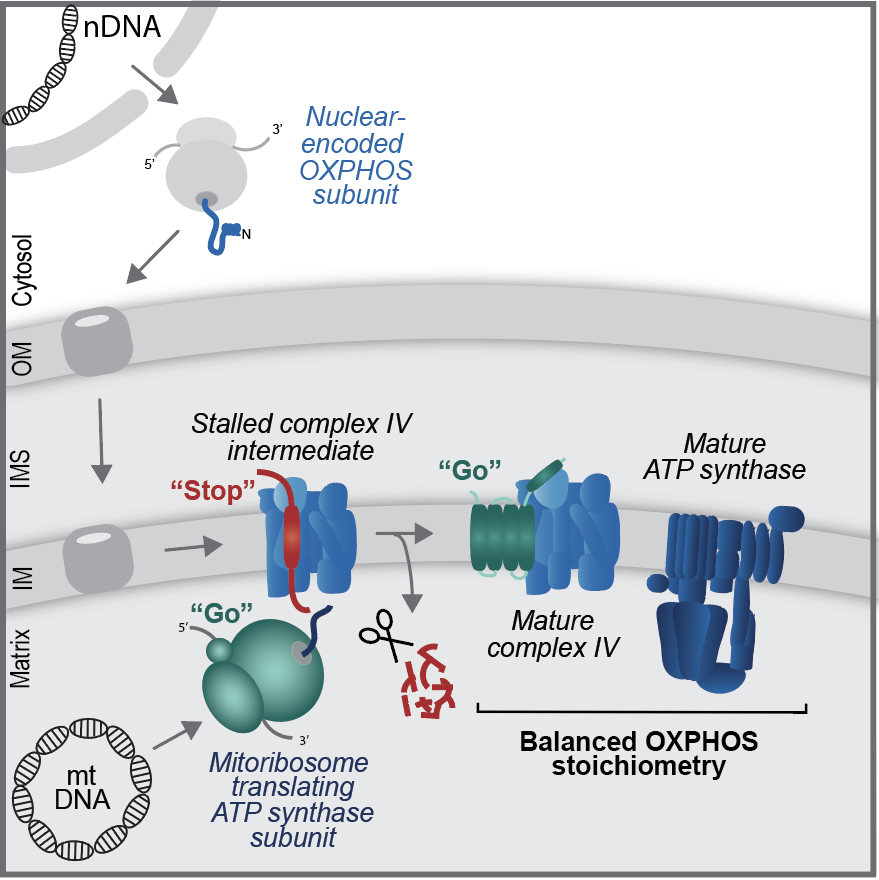
Synchronized assembly of mitochondrial protein complexes
A universal cellular feature is that complex biological functions are performed by multi-protein assemblies that act as molecular machineries. The majority of mitochondrial proteins do not act as single entities, but assemble into larger protein complexes. Technological advances have identified the subunit composition of a large number of these mitochondrial protein complexes. However, how the correct stoichiometry of the well-defined various subunits is achieved during biogenesis of multimeric protein assemblies remains elusive. We are interested how the stoichiometry of protein complexes is regulated during their biogenesis and are investigating the assembly process of the mitochondrial oxidative phosphorylation system.